Bodies with a crystalline structure are composed of groups of simple elements (called "Weiss enclosures" formed in turn by molecules joined by forces of 'ionic' attraction.
The molecules of these crystalline bodies have a specific orientation and consequently of their atoms, are very stable and therefore with a minimum amount of energy. The result is a very definite shape and volume according to the different systems of crystallization.
Keep in mind that although this energy is very small, there are always electric fields between their reticular enclosures.
Electric fields are governed by Coulomb's Law, which basically says:
• Forces of attraction or repulsion between two particles are directly proportional to their ionic charges (known as chemical valences).
• This force is inversely proportional to the distance between the particles (which is why proximity or approach increases the forces of attraction or repulsion).
• It is also inversely proportional to a factor called "dielectric constant of the medium." This factor is of great importance in our case because our purpose is to modify the dielectric constant of water.
Which means that the challenge is to create an electro-physical field to modify the characteristics of the water molecule.
We will now analyze the characteristics of a water molecule:
WATER DIPOLE
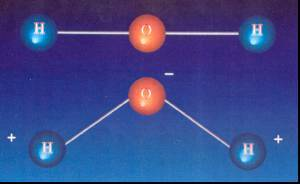
- The molecule of normal water (H2O) is is made up of two hydrogen atoms (H) and one oxygen atom(O), chemically bound.
- The bond between the hydrogen and oxygen atoms has a defined and constant distance and orientation.
This physical study was conducted based on those conducted by Dr. Carmelo Hoyos Fitto, Dr. José Ignacio Isusquiza Carro and Dr. Jesús Piernas Manzano; and published in the book entitled "RENAL LITHIASIS " (ISBN 84-500-5204-1).
ORDINARY WATER ATOM
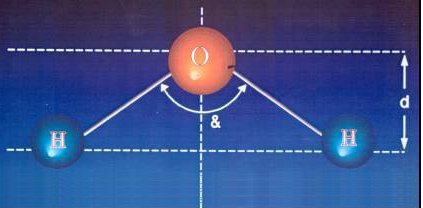
& = 105º
• The water molecule is not symmetrical, and its asymmetry is precisely why it constitutes a dipole (+/-) that has a permanent dipole moment.
• The dipoles have the property that when placed in an electric field, they are oriented by aiming their negative charge to the positive field and the positive field to the negative thereof.
• The result is that a dipole or dielectric decreases the attraction between charges of different signs.
The consequence is that these dipoles with a high dielectric constant, such as water, decrease the forces of attraction between the molecules that form crystals, and the capacity of combining their atoms (H and O) produces the dissolution of many bodies, especially salts.
Which means that water itself has a great ability to dissolve crystals, thanks to its high dielectric power.
With the SLACKSTONE II® system, our purpose is to further increase water's ability to dissolve in order to dissolve salts with crystals of high cohesion and low solubility.
This is achieved by increasing the dipole moment, in other words, the asymmetry of the water molecule.
It is therefore necessary to influence the distance and placement of the H and O atoms. If we separate the O atom from the H atoms, and decrease the angle of spatial positioning of these compared to the angle, the dipole moment would increase.
DEPOLARIZED ATOM OF "Dyalitic Water"
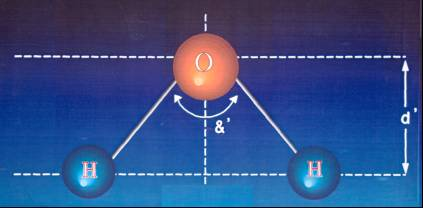
&' =< 105º
If we compare the two preceding figures, we can observe that:
| ORDINARY WATER ATOM |
DEPOLARIZED OF Dyalitic Water |
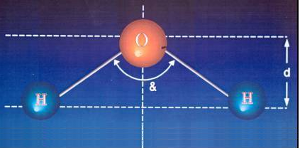 |
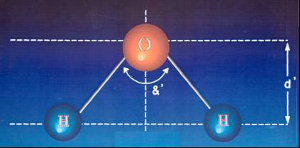 |
| & = 105º |
&' =< 105º |
• angle &' is less than &.
• the distance between the H atoms relative to O atom: d' is greater than d.
|
This new position of its atoms gives water, already transformed into Dialytic Water:
• Increased dipole moment (more energy).
• Increased dielectric constant.
• More power to reduce the cohesive forces of other crystalline elements.
• Increased capacity to dissolve mineral salts.
|
How is this achieved?
We require an energy to change the position of the atoms constituting the H2O molecule.
If we provide a powerful external energy (i.e., electricity) we run the risk that the effects are so strong as to destroy the molecule itself (electrolysis).
We need energy to modify the molecule, but without destroying it, just enough to slightly "dislodge" the H atoms relative to the O atom.
|
This energy is obtained through the patented process of the SLACKSTONE II® system
|
The System is presented in a hermetically sealed glass ampoule (two per package), in which there are macro crystals of a certain size, sodium chloride and lithium chloride, properly prepared and treated with cinnamic aldehyde, with a high dipole moment to facilitate energy transmission through the glass of the ampoule.
The union of these crystals causes the generation of an energy of a different intensity according to the positioning of the crystals and, therefore, of their atoms.
This energy, which we call electrostatic, is produced between different crystals, forming small electrostatic fields whose energy is transmitted perpendicularly to their crystalline tables and facets.
It is therefore very important that these crystals be a certain size, for if they were like dust, the fields would be very numerous but small, and if they were large, the contact would be smaller and the distance (which decreases the energetic power) would be too large.
The glass ampoule used in the SLACKSTONE II® System is not the only way of putting that energy into contact with the water to be modified, but it proves to be the most appropriate way to enclose sodium and lithium crystals that create hundreds of small electrostatic fields, which together generate a circular area around the ampoule. Furthermore, the glass used in the manufacture of ampoules SLACKSTONE II® System possesses special features and allows the passage of that energy.
DIELÉCTRIC EFFECT

The ampoule of the
SLACKSTONE II® System, placed in direct contact with the water for at least 24 hours, makes the electrostatic energy it produces to modify its dipole, slightly altering the position of its atoms and charging it in turn with dielectric or dipole energy, capable of dissolving and disintegrating other crystalline bodies, for example, barely soluble calcium salts.
This Dialytic Water, so named for its disintegrating power, is obtained by purely physical methods.
This potential energy is also slightly radioactive, as tested by a Geiger-Muller counter on both the ampoule and the treated Dialytic Water.
Dialytic Water's greater capacity to solubilize salts has also been proven in the laboratory.
Dialytic Water prepared with the SLACKSTONE II® System does not alter its chemical properties but only its physical structure (the position of its atoms).
In this regard, we include the study performed in France: "Difference between Normal Water and Dialytic Water using Vincent's Bioelectronic Technique".
|
DIFFERENCE BETWEEN NORMAL WATER AND DIALYTIC WATERAPPLYING VINCENT'S BIOELECTRONICS TECHNIQUE
La Bioelectrónica es una técnica elaborada en 1945 por Louis Claude VINCENT, ingeniero hidrólogo. Permite caracterizar una solución, un producto o bien un organismo vivo según diferentes parámetros: el pH, el factor de óxido reducción (rH2) y la resistividad (rho). Esta técnica puede por tanto utilizarse principalmente para estudiar la calidad de las aguas de bebida.
El pH mide la concentración en iones H+ de la solución y representa el carácter magnético así como la capacidad energética del medio. En lo que respecta al factor de óxido reducción, mide la concentración en electrones y representa la capacidad reguladora del medio. Finalmente, el factor de resistividad mide la concentración en electrólitos.
Seguidamente, los resultados de los análisis efectuados el 20 de noviembre de 2008 en el agua Montcalm y un agua puesta en contacto con el sistema SLACKSTONE II® durante 24 horas.
1. Montcalm Water 2:
• Parameters measured in the laboratory:
- Potential: E = 247 mV / ref. AgCl
- pH = 6.07
- Resistivity factor: p = 23 300 ohm / cm3
• Parameters deducted:
- Conductivity factor: σ = 43 mS
- Dry residue index: TDS = 31 mg / L
->Oxide reduction factor: rH2 = 27.2
For comparison purposes, the figures published on the label of the bottle Montcalm:
ρ = 30 000 ohms
TDS = 28 mg / L
rH2 = 25.95
This water is registered in the list of waters classified as "perfect".
2.- Montcalm Water(*) in contact with SLACKSTONE II® system for 24 hours:
• Parameters measured in the laboratory:
- Potential: E = 288 mV / ref. AgCl
- pH = 5.9
- Resistivity factor: ρ = 20 400 ohm / cm3
• Parameters deducted:
- Conductivity factor: σ = 49 mS
- Dry residue index: TDS = 35 mg / L
-> Oxide reduction factor: rH2 = 28,2
(*)Montcalm water: Mineral water from the spring of the same name, located at 1,100 meters above sea level in the Valley of Auzat (Midi-Pyrénnées Region, France).
3. Analysis of results:
Comparing the results of the two solutions we can observe that the SLACKSTONE II® System lowers the pH of the solution, which means that the medium would become richer in protons and thus increase its magnetic ability.
Also, the resistivity is weaker in the solution put into contact with this system, and therefore, the solution would be richer in ions.
Finally, as regards the reduction factor oxide, it is slightly higher after being in contact with the SLACKSTONE II® System. However, this increase is very weak and unrepresentative.
In terms of water quality, the following indicators of a good quality are: a resistivity greater than 6000 ohm / cm3, a pH between 5.5 and 6.9 and an oxide reduction factor ranging between 24 and 28.
It would be interesting to perform further testing, mainly on different waters, but considering the above results the SLACKSTONE II® System does not appear to alter the good quality of the water in any way.
|
L.G. (por encargo de SODALITE, Francia.)
Extension of data referred to the Physical Basis (*)
This procedure, patented and registered under the name
SLACKSTONE II® is completely natural and is based on the properties of the solid state of matter and in particular its crystalline state.
The crystals are formed by clusters of simple elements (called "Weiss enclosures") formed in turn by molecules linked by 'ionic' attractive forces, "cohesive" forces of secondary valences (Van der Waals), etc.
These systems require an orientation of the molecule and consequently a very stable atomic distribution and minimum energy, according to the number of electrons and the corresponding "spins" of the atoms. The result is a volumetrically predominant form, known as "crystallization systems", such as the cubic, hexagonal, monoclinic, triclinic system, etc., with different properties according to the inclination, for example, of a light beam, an effort, etc., made with respect to the "axes of crystallization" of the crystalline body.
In this regard, the ionic physical forces, cohesion, etc., indicated above are significant in a relatively large set, but small in the individuality of each atom and molecule and, therefore, if we "attack" these molecules with a system of "action of immediacy" (i.e., individualized action), we can achieve disintegration, or rather the disaggregation of crystalline aggregates.
Moreover, in these crystalline aggregates the molecules that form the edges and external corners of said crystal aggregates are (so to speak) more defenseless, and therefore, these outermost parts can easily be disaggregated so that the crystalline elements with their blunt vertices and reduced length ensue, as well as the apparent volume of the aggregate.
(*) In this regard see the book entitled "CAMPOS ELÉCTRICOS Y MAGNÉTICOS" de J.I. Martín-Artajo (Editorial Aguilar, Madrid 1984).
All these forces of attraction between molecules of a crystal depend on the medium in which the crystal is found.
It has not been possible to express the value of these (attractive and cohesive) forces by mathematical formulas, but for our purpose it is enough to know that these forces decrease proportionally as the "dielectric coefficient" of the medium increases (e).
An approximate formula for certain physical conditions is accepted as the value of the electric field of an electric point load Q1 in the distant point P2 (r12) can be expressed as follows:
And the resulting mechanical force on a charge (point) Q2
If instead of a point load we had a "dipole moment" polarized body consisting of two masses (+q) and (–q) to the mutual distance D, it would be in the direction of the bipolarization axis (cosO=1):
The term
(qD) is the so-called "dipole moment" and its value depends on the nature of the bipolarized body (water, glycerin, nitrobenzene, ethyl alcohol, etc.) enunciated in descending order.
According to this theory of the solid and crystalline state of matter, we believe that (having knowledge of physics) this extension of the basis of Professor Dr. D. Jose Ignacio Martin-Artajo Alvarez is well understood.
As a second extension of the data of the "Physical Basis" of the
SLACKSTONE II® System to prepare
Dialytic Water, and in response to various queries made by some health professionals, starting from the 6th edition we have included the following additional information.
But first we should note that it is necessary to carefully read Chapter 3, especially the section "Physical Basis".
1.- SOBRE LOS COMPONENTES DE LA AMPOLLA
1.- ABOUT THE COMPONENTS OF THE AMPOULE
The presence of sodium chloride and lithium chloride in the SLACKSTONE II® ampoules is mainly due to the similarity of their properties:
• Both are alkaline metals belonging to Group I
• They are very reactive and good conductors of electricity
• They have much affinity:
| Propiedades (algunas) |
NA |
LI |
| Estado |
Sólido |
Sólido |
| Estructura cristalina |
Cúbica centrada |
Cúbica centrada |
| Estado Iónico |
4, 6 |
4, 6 |
| Energía de Ionización (kJ.mol-1) |
494 |
519 |
| Afinidad Electrónica (kJ.mol-1) |
+53 |
+60 |
| Electronegatividad (escala Pauling) |
0.93 |
0.98 |
| Entalpía de Fusión (kJ.mol-1) |
2.64 |
4.6 |
| Punto de Fusión (ºC) |
97.8 |
180.5 |
| Punto de Ebullición (ºC) |
883 |
1342 |
| Densidad (kg/m3 20ºC) |
971.2 |
534 |
| Radio Atómico (pm) |
191 |
152 |
| Radio de Van der Waals |
0.0 |
0.0 |
| Estado de oxidación |
-1. +1 |
-1. +1 |
|
Comparten valencias en sus átomos
(Tienen la misma configuración electrónica en la última capa)
|
Na) Yd-) 8e-) 1e- |
Li) 2e-) 1e- |
Minerals are prepared using an exclusive and proprietary microencapsulation process (one of the objects of patents of the
SLACKSTONE II® ampoule).
An interesting fact is that the sodium chloride (rock salt, halite) we use has its origin in the Miocene period (10-15 million years ago). It is known as "mirror salt" for its purity and transparency.
Moreover, due to its high dipole moment, cinnamic aldehyde is used as an enhancer in the transmission of the action (electrostatic energy) of said minerals to water. It is placed as a cord that must unite the north and south poles of the ampoule.
The presence of sodium chloride, lithium chloride and cinnamic aldehyde in the composition of the SLACKSTONE II® ampoule is solely due to its physical properties as a whole, and not to chemical properties. Our system is a purely physical process.
If we consider these components exclusively by their chemical action, we would be making a grave error, since their uses are diverse:
Uses of Sodium
• An essential component of the extracellular space of living beings.
• In food it is used as an essential nutrient, food preservative, condiment (chloride).
• Coolant (heat exchanger) in nuclear reactors.
• Manufacture of anti-knock in gasoline.
• Reducer in obtaining other metals.
• Detergents, bleaches, manufacture of paper and textiles.
• Street lighting.
• Fertilizers (in the form of nitrate).
• In optics and as a fixing agent in photography.
• It was used as payment to the Roman legionaries (salary).
Uses of Lithium
• In medicine, as antidepressant medication (carbonate).
• Manufacture of lubricating greases (stearate).
• Fuels, very hard alloys, battery electrodes (anodes).
• Ceramics and special glasses.
• Coolant.
• Moisture absorbent in air conditioning (bromide and chloride).
• To inflate life jackets and rocket fuel (hydride).
• Air purification and ventilation systems in submarines4 and spacecraft, to remove carbon dioxide (hydroxide).
Uses of Cinnamic Aldehyde
• Making flavors for the food and pharmaceutical industry
• Preparation of cosmetics and perfume fragrances.
• As a stimulant of the digestive function.
2.- ABOUT THE AMPOULE'S GLASS
On the one hand, the glass vial must also meet special characteristics (see table), to allow the passage of this energy, without loss or variables, and on the other, the process also has to involve the refraction of light (Maxwell Equations).
The SLACKSTONE II® ampoule is hermetically sealed and as a whole we can consider it as a cell or battery.
Therefore, its components never come into contact with the water to be treated and the emission of energy into the water occurs only through the glass of the ampoule.
In the event the ampoule is accidentally broken, it would no longer be useful for the process and must be replaced with a new one. If inadvertently ingested, the resulting liquid has a strong salty and bitter taste. Continued ingestion of the solution would result in a digestive disorder (diarrhea).
The lithium contained in the ampoule (as chloride) does not exceed 500mg. We note that for the medicinal use of lithium (as carbonate) in antidepressant treatments, the recommended daily dose is 600 mg
| |
 |
|
3.- ABOUT THE SLACKSTONE II® SYSTEM
First and foremost, we must keep this concept in mind:
Water is known as "the universal solvent"
because it is the liquid that dissolves the most substances
Since water has bipolar molecules, it is a great medium that dissolves ionic compounds, such as mineral salts.
Water in its normal state (105° angle dissolves non-ionized and ionized hydrophilic solutes with low and high molecular weights by itself as perfect solutions and suspensions based on its dielectric constant, dipole moment and the concentration of solutes. When these crystalline bodies are hydrophobic and amphipathic, ionic cohesion forces (Van der Waals), attraction forces intervene between the molecules and crystalline elements (Weiss enclosures), etc.
Water covers all the molecules (in our case the molecules of the stones, gravel and micro-crystals present in the body and anywhere they are found) because of their polarity and the hydrogen bridges or non-covalent joints formed with molecules, parts of molecules and ions.
The greater the dipole moment (higher energy)
the greater the solubility of the water
Through the
SLACKSTONE II® ampoule, normal water is subjected to electronic radiation of slightly active alkaline salts for a period of 24 hours (the maximum distancing between the water molecules occurs within 24 hours, as well as the maximum increase of the set of turns, spins). A modification of the molecular arrangement of water which results in
Dialytic water is then produced by physical action.
With regard to normal water, Dialytic water has a higher dipole moment (higher energy), higher dielectric constant, greater power to reduce the cohesive forces of crystalline elements and a greater capacity to dissolve mineral salts.
Dialytic water, once incorporated into the body and by an action of immediacy, progressively and cumulatively weakens the ionic bonds of the crystalline agglomerates formed (as previously noted) by ionic cohesive forces (Van der Waals), forces of attraction between molecules and crystalline elements (Weiss enclosures), etc.
This action disaggregates the molecular layer of these agglomerates and detaches the most vulnerable parts, such as peaks and edges. When the agglomerates are large, progressive fragmentation occurs.
The SLACKSTONE II® System (the greater energy emitted by the ampoule) is based on the interface electropairs (in this case, glass-glass). These electropairs depend on the movements of electrons of one or another alkali metal, with alternations of approximation and distancing of electrical charges and their corresponding spins.
Energy emission of the SLACKSTONE II® ampoule can be measured by various methods, but perhaps the most spectacular is contained in the "Report on Kirlian photographs." This study was not done by us.
4.- ABOUT THE ENERGY IN THE SLACKSTONE II® AMPOULE
The electrostatic energy of the
SLACKSTONE II® ampoule is only discharged in the presence of water and is sufficient to prepare 40 glasses of
Dialytic Water. After that time you need to replace the ampoule with a new one.
The energy transmitted to the water is temporary and lasts while the charged field (the ampoule) is maintained sufficiently close to the water to be treated. For this reason we must ingest the Dialytic Water immediately after preparing it (see package insert), a process that takes about 24 hours, enough to modify all the molecules of the recommended water mass (250cm3).
For this reason, prepared Dialytic Water cannot be packaged, as it has been suggested to us many times.
When you extract the source of energy from the water (the SLACKSTONE II® ampoule), the modified molecular position returns to its initial position (the 105°angle), but this does not happen instantly.
Ingested Dialytic Water is metabolized by the body in 30-45 minutes, which is sufficient time for its modified molecules to not be transformed again into normal water.
The mass of the ampoule is directly related and proportional to the mass of water to be treated.
The SLACKSTONE II® ampoule has no other purpose than to be the means of transforming normal water into Dialytic Water.
Dialytic Water has properties to eliminate and / or prevent the formation of crystalline bodies in the body (all kinds of stones, gravel, micro-crystals, etc., wherever they are).
The SLACKSTONE II® System to prepare Dialytic Water is sold (since 1966) in boxes of 2 ampoules (for 40-80 days of treatment) in several countries.